Cellular interactions ensure the harmonious growth and optimal function of tissues and organs. Invariably, cancer cells adapt and escape these strict regulations to proliferate and invade surrounding or distant tissues. Using complementary approaches and experimental models, the 'Epithelial growth and cancer' team is seeking to understand how short- and long-distance signalling are integrated in epithelial cells and how their alterations contribute to tumour development. More specifically, we are studying
1. the role of the Hippo tumour suppressor signalling pathway, and how it is regulated
2. cell competition and its influence on tissue and tumour composition
3. interactions between the tumour and the body, particularly during cachexia.
Project 1: Hippo Signaling Pathway (L. Heron-Milhavet / A. Djiane)
The Hippo signaling is a tumor suppressor pathway often deregulated in cancers. We have shown in Drosophila that the apical protein Bbg regulates the subcortical actin cytoskeleton and participates in the activity of Yki, the terminal transcription factor of the pathway (homolog of YAP and TAZ; Forest et al., J. Cell Biol. 2018). We have also shown that the apical protein MAGI1 regulates the activities of the Hippo pathway and the P38 pathway in luminal-type breast cancer cells (Kantar et al., Sci Reports 2021).
We are currently studying the link between the Hippo and P53 pathways in chemotherapy resistance in colorectal cancer.
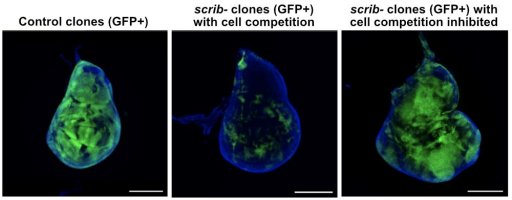
Project 2: Ecological Relationships Between Cells: Cell Competition (P. Lassus / A. Djiane)
We are interested in the phenomenon of cell competition, which results in the elimination of weaker (loser) cells by stronger (winner) cells. This mechanism allows for tissue optimisation and serves as an anti-tumour mechanism to locally eliminate pre-cancerous cells. Cell competition has also been implicated in tumour evolution and the selection of the most aggressive subclones.
Through various omics approaches (RNA/protein/metabolite) and genetic screens, we aim to identify new regulators of cell competition and stress response pathways that enable the elimination of pre-neoplastic cells.
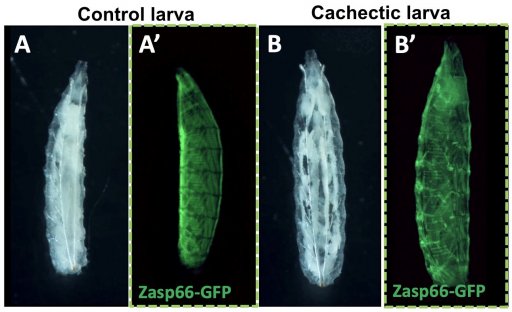
Project 3: Inter-organ Communication During Cancer-associated Cachexia (C. Géminard / P. Senesse / A. Djiane)
We have developed models of cachexia in Drosophila larvae in response to tumours confined to wing imaginal discs. These models reproduce the hallmarks of cachexia: fat tissue atrophy (the white tissue in the figure below), muscular disorganization (visualized by the live marker Zasp66-GFP), hyperglycemia, and inflammation. Using these original models, we aim to identify the messages exchanged between the tumour and the affected organs that mediate cachexia. In parallel, we are using classical murine models of cachexia (C26 colon tumours and KPC pancreatic tumours), and patient samples to test the clinical relevance of our findings.